VisionGate Presents at the 2018 conference of the American Thoracic Society: Positive Rate Of The Lung Cell Evaluation (LuCED) Test For Patients With Chronic Obstructive Pulmonary Disease (COPD)
Positive Rate Of The Lung Cell Evaluation (LuCED) Test For Patients With Chronic Obstructive Pulmonary Disease (COPD):
Authors: Meyer, Kelbauskas, Nelson, Zulueta
Control No: 2018-LBA-17183-ATS
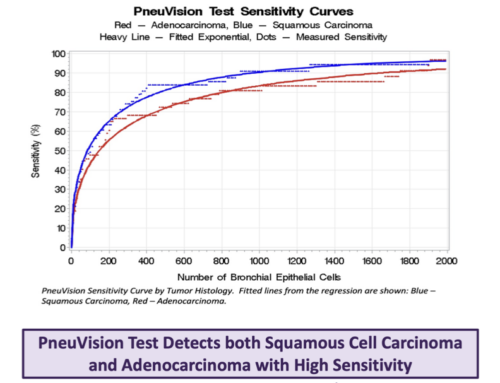
Background: The LuCED test is based on analysis of sputum by the Cell-CT platform that computes 3D images of cells with isometric resolution, allowing orientation-independent measurements of 704 3D structural biomarkers to generate a probabilistic score to identify abnormal cells. These cells are confirmed as abnormal through cytopathology review. Published studies of LuCED accuracy show 92% sensitivity and 95% overall specificity for biopsy confirmed cancer that was consistent by tumor histology and stage. The figure shows cross-sectional views of several lung cells as imaged by the Cell-CT. This abstract focuses on the way COPD influences LuCED positive rate. The LuCED test incorporates a criterion (SAT criterion) to define a satisfactory specimen (450 normal bronchial epithelial cells). Recent findings (Prindiville, Cancer Epidemiol Biomarkers Prev 2003;12:987-993) indicate prevalence of pulmonary dysplasia in 30% of patients with COPD that are understood to be free of cancer. The LuCED test is trained to recognize the spectrum of cell abnormality from squamous atypia and atypical adenomatous hyperplasia through cells from non-small cell cancers. Dysplastic cells exfoliate from lesions into sputum and therefore LuCED should detect these cases. Here we test the hypothesis that the LuCED positive rate is approximately equal to the dysplasia prevalence for COPD patients.
Methods: Specimens from 16 normal patients without COPD and 23 specimens with COPD or smoking history exceeding 30 pack-years were processed by LuCED. Positive rate was computed as the percentage of cases with abnormal cells divided by the number of cases exceeding the SAT criterion.
Results:
Positive rate for disease free patients was 6%. Positive rate for patients without lung cancer but with COPD was 26%.
Conclusions:
Differential detection of abnormal cells for patients with COPD as compared with disease free patients conforms with published dysplasia prevalence in patients with COPD. These results suggest that the LuCED test may have utility in the identification of pre-cancerous lung conditions.