VisionGate Presents at the World Conference on Lung Cancer for 2016 in Vienna: Non-Invasive LuCED® Test for Endobronchial Dysplasia, Enabling Chemoprevention Therapy with Drugs Such as Iloprost
The Non-Invasive LuCED® test for Detection of Early Stage Lung Cancer
Author Block: Meyer, Bell, Sussman, Wilbur, Presley, Hayenga, Lakers, Reyna, Davies, Field, Yang, Lancaster, Zulueta, Nelson
Background
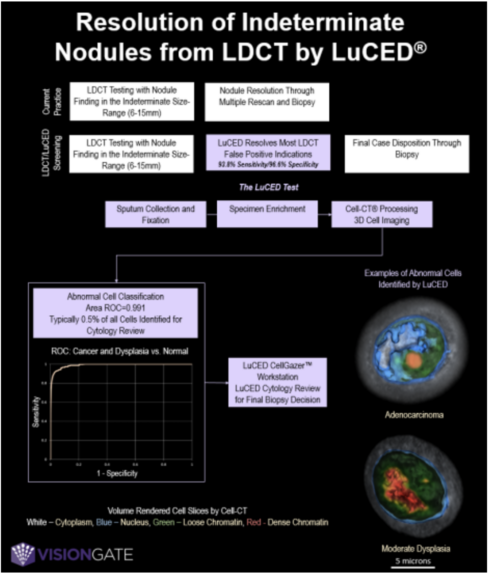
LDCT screening for lung cancer often triggers follow-up scans for indeterminate nodules. The non invasive LuCED test for detection of early stage lung cancer may resolve nodule findings and reduce LDCT false positives. In LuCED, patient sputum is analyzed by the Cell-CT® which computes 3D images of single cells allowing measurement of 3D structural biomarkers to identify potential abnormal cells. Final case disposition is determined through cytology review of these cells. Example images of abnormal cells identified by LuCED are shown in Figure 1.
Methods: Sputum samples from 127 patients were processed by LuCED: 65 patients had biopsy-confirmed lung cancer; and 62 patients were normal controls. Sensitivity was computed as the percentage of cancer cases where abnormal cells were found by LuCED. Generally, abnormal cells found in a case otherwise understood to be normal could constitute a diagnostic overcall and counted as a false positive. However, a finding of abundant (>5) abnormal cells in cases understood to be normal indicates discovery of a possible occult cancer or dysplastic lesion. Accordingly, these cases were not included in specificity calculations.
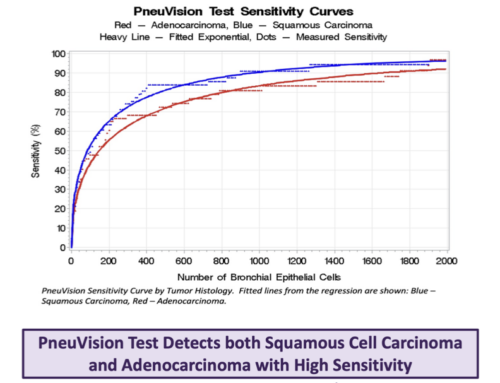
Results: For cancer cases, the histology included adenocarcinoma (29 cases), squamous cancer (24), small cell lung cancer (5) and undifferentiated cancer (7); representing stages 1 (14), 2 (11), 3 (25), 4 (14), and unknown (1). Abnormal cells were found in 61 of 65 cancer cases for sensitivity of 93.8%. For stage 1 and 2 cancer, sensitivity was 88%.
Ten cells exhibiting changes consistent with atypical adenomatous hyperplasia were found in one case. After removal, there remained two false positive cases, leading to specificity of 96.7% (N = 61).
Conclusions: The LuCED test demonstrates accurate detection of early stage lung cancer with the potential of detecting pre-cancerous conditions of the lung. Results suggest that suspicious nodules may be efficiently reconciled by LuCED when used adjunctively with LDCT.