VisionGate Presents at the World Conference on Lung Cancer for 2009 in San Francisco: The Lung Cell Evaluation Device (LuCED): Early Detection of Lung Cancer in Sputum Based on 3D Morphology
The Lung Cell Evaluation Device (LuCED): Early Detection of Lung Cancer in Sputum Based on 3D Morphology
Abstracts 13th World Conference On Lung Cancer 43
Meyer, Michael G.; Patten, Florence W.; Neumann, Thomas; Hayenga, Jon W.; Steinhauer, David E.; Rahn, John R.; Nelson, Alan C.
VisionGate, Gig Harbor, WA, USA
Background: The Cell-CT produces 3D volumetric cell representations in isometric, sub-micron resolution based on computed tomography. VisionGate, Inc., in collaboration with the University of Washington, is developing the LuCED test to score sputum samples processed by the Cell-CT for evidence of cell dysplasia or cancer. LuCED scores may be used clinically to assist in patient triage.
Methods: The LuCED test comprises a series of steps starting with cell preparation including fixation and stain- ing with hematoxylin. Cells of each type found in sputum from normal patients were volumetrically sampled, as were cancer cells from culture and isolated from resected lung tissue. The 3D volumes were processed to extract features that correlate with the abnormal condition of the cell. LuCED scores were trained using cytological diagnosis as the reference. Training was done in a rigorous, cross-validated mode, using a genetic algorithm for feature selection and adaptively-boosted logistic regression to produce classifiers. LuCED score performance was assessed using receiver operator characteristic (ROC) analysis. Variance in ROC results were assessed using boot- strap techniques. Prevalence counts of abnormal cells in sputa from patients with confirmed cancer were used to estimate patient detection rates based on cell-classification performance.
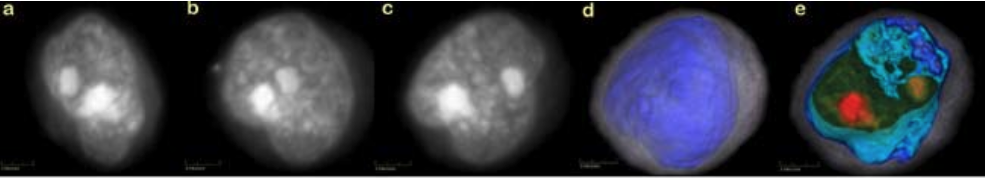
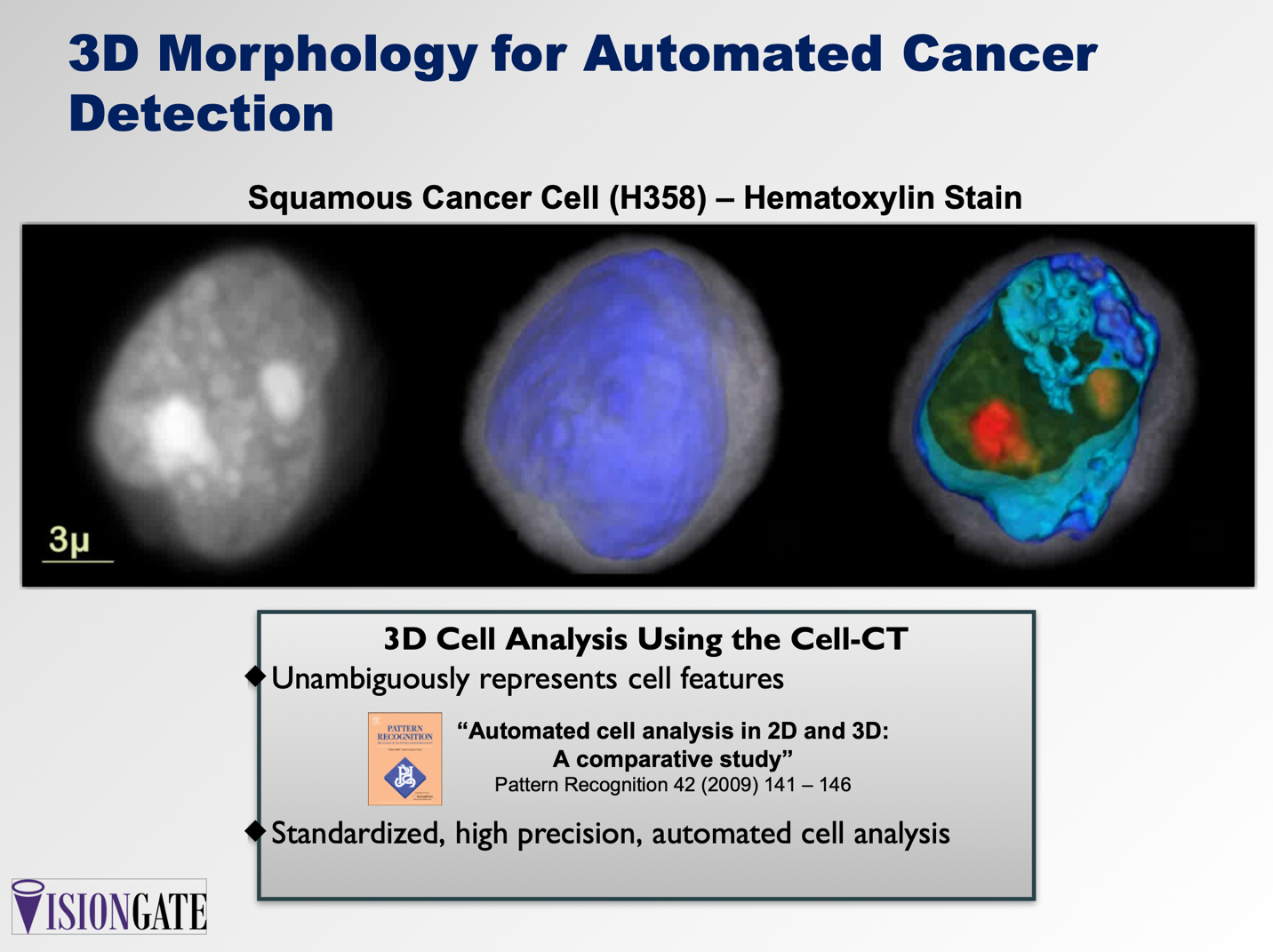
Results: Movies of rotating volumes were produced for a variety of cells and show the distinctive 3D morphol- ogy of each cell type. Figure 1 shows 2D maximum-intensity projections in different orientations, created from a 3D reconstruction of a squamous-cancer cell (a-c). Figure 1 also shows the same cell from the perspective in (c), whole and cropped, where coloring and opacity have been adjusted to enhance cell features. In (d) and (e), cytoplasm is in translucent gray, the nuclear wall is in opaque blue and the nucleoplasm is rendered in a green to red gradient, with nucleoli in opaque red.
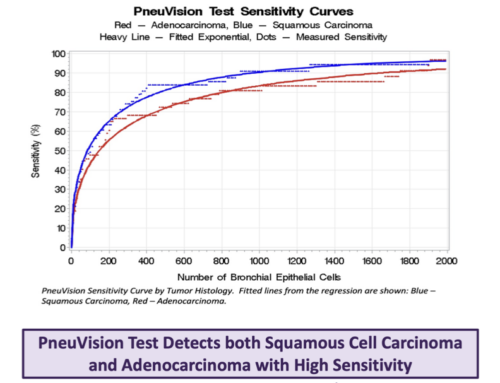
Normal cell versus cancer training on 3D data sets produced an ROC curve showing near-perfect discrimination. Analysis of sputa from patients with cancer shows that 75% have abnormal cells. Specimen-based detection rates depend on the number of abnormal cells in the specimen. Based on cellular prevalence counts, we esti- mate that LuCED sensitivity exceeds 90% as specificity approaches 100% for patients with cancer cells in sputum.
Conclusion: Cell analysis in 3D provides an unobstructed and unambiguous representation of normal and cancer cell morphology making the LuCED score an exceptionally accurate means for automated assessment of lung cancer risk based on patient sputum.