VisionGate Presents at the 2014 conference of the American Society for Clinical Oncologists: Early detection of lung cancer based on three-dimensional, morphometric analysis of cells from sputum
Data to be Presented at ASCO 2014 Demonstrates that Non-Invasive LuCED Test Detects Stage I and II Lung Cancer
PHOENIX, Ariz. – May 15, 2014 – VisionGate Inc., an in vitro diagnostics company that is developing a revolutionary, non-invasive test for the early detection of lung cancer and other applications, announced that positive results from a clinical study of its LuCED lung test will be highlighted in a poster presentation (Abstract #7547) during the American Society of Clinical Oncology (ASCO) 2014 Annual Meeting at McCormick Place in Chicago on Saturday, May 31, 1:15 to 5 p.m. in S Hall A2, poster board 155.The study demonstrates three major advantages to incorporating the LuCED lung test into the diagnostic algorithm: high sensitivity, exceedingly high specificity and the ability to detect cancer cells in stages I and II.
“Early detection of lung cancer is key – there’s an immense need for safe, effective early detection options to help increase patient survival rates,” said Harvey Pass, M.D., Division Chief of General Thoracic Surgery and NYU Langone Medical Center.
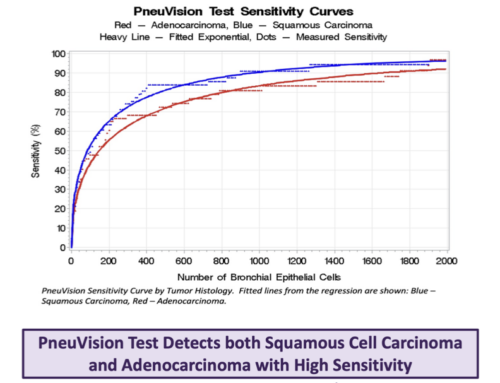
For the study, investigators used VisionGate’s LuCED test with the Cell-CT® cell imaging system to analyze sputum samples from patients with biopsy-confirmed, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The results show that VisionGate’s Cell-CT accurately detected lung cancer cells in three-day pooled, spontaneously produced sputum.
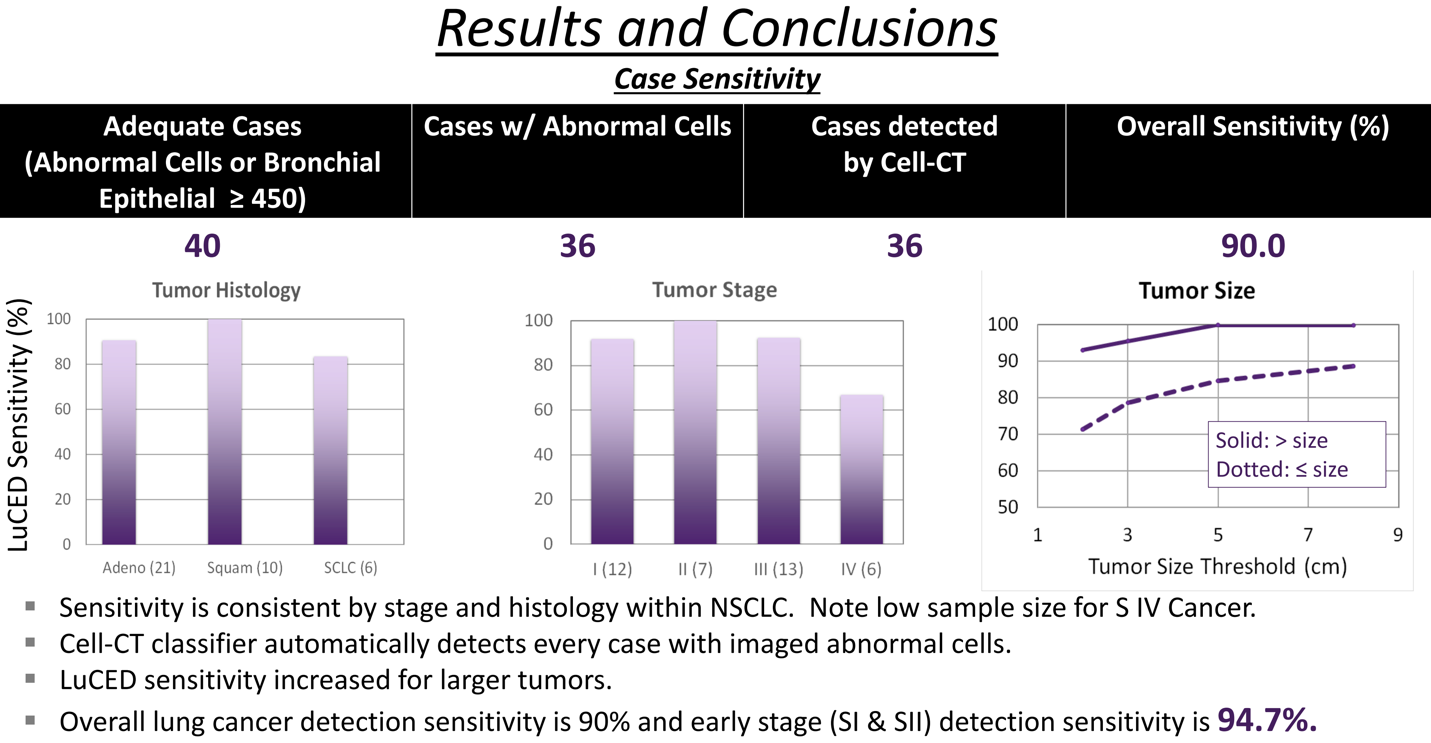
In the 30 cases studied, 26 were NSCLC and four were SCLC. Moderate/severe dysplasia or cancer cells were found in 28 of the 30 cases. Half of these cases were either Stage I or Stage II lung cancer with 93.3% sensitivity to early lung cancer.
Importantly, among 5,146 sputum cells from 17 normal patients at high risk for lung cancer, the Cell-CT processed all cells with zero false positive indications for highly specific lung cancer screening. Based on this performance, the lower 95% confidence interval bound for cell specificity is 99.8%.
Today, lung cancer is by far the leading cause of cancer death among both men and women, and more people die of lung cancer than of colon, breast, and prostate cancers combined.
“While CT scans are effective at identifying suspicious lesions, more invasive procedures are required to confirm malignancy and the type of lung cancer,” Bonnie J. Addario Lung Foundation’s Bonnie Addario said. A safe, accurate, non-invasive diagnostic test that could be used earlier in the process would be a significant game changer for the diagnosis of lung cancer. Although early stage lung cancer is typically asymptomatic, the ability to detect it sooner at stage one or two offers the patient a much improved outlook for longer-term survival.”
VisonGate’s LuCED test incorporates its Cell-CT automated system, which harnesses the power of cuttingedge optics and computational technology. It has the capability to capture images very rapidly, rendering scanned objects into 3D digital images. For this particular study, the Cell-CT platform produces clear, detailed, 3D images of cells in sputum, which the system automatically analyzes to identify key features, or biosignatures, associated with potential malignancy. The analysis yields a high score when cancer cells are present.
“Our breakthrough technology platform, the Cell-CT, has demonstrated exquisite sensitivity to rare cancer cells in sputum with exceptionally low false positives, making this the first viable non-invasive, non-radiation test for lung cancer,” VisionGate Founder and Chief Executive Officer Alan C. Nelson, PhD said. “In essence, we’ve added another dimension to pathology by removing human error to overcome hurdles traditionally seen with sputum testing. This is a significant milestone for VisionGate.”
VisionGate is in the final phases of its Clinical Laboratory Improvement Amendments (CLIA) certification and plans to open a best-in-class clinical services laboratory in August 2014 in Phoenix, Arizona. One of the state’s most supported ventures, it was awarded the Arizona Governor’s Celebration of Innovation Award for Innovator of the Year – Start-Up Company in 2011.
About VisionGate
VisionGate, Inc. is led by Dr. Alan Nelson, physicist, bioengineer and entrepreneur who developed the world’s first and only automated screening test to detect cervical cancer, marketed today as FocalPoint by Becton Dickinson. VisionGate offers the first automated 3D cell imaging platform, the Cell-CT, which computes highresolution 3D biosignatures from intact cells. The company’s first implementation of the Cell-CT is the LuCED test, initially being developed as a CLIA lab developed test for adjunctive use with low dose x-ray computed tomography (LDCT) screening for the early detection of lung cancer in high-risk individuals. Adjunctive use of LuCED to better manage the high rate of false positive results in CT screening could increase the utility and cost effectiveness of the approach, which has been shown to decrease lung cancer deaths in high-risk patients. VisionGate is headquartered in Phoenix, Arizona, and has a research and development office in Seattle, Washington. It currently holds 106 issued patents. For more information about VisionGate, visit www.visiongate3d.com.
About Lung Cancer
According to the American Cancer Society, lung cancer is by far the leading cause of cancer death among both men and women. Lung cancer (both small cell and non-small cell) is the second most common cancer in both men and women (not counting skin cancer). In men, prostate cancer is more common, while in women breast cancer is more common. The American Cancer Society estimates that in 2014 in the United States about 224,210 new cases of lung cancer (116,000 in men and 108,210 in women) will be diagnosed, and an estimated 159,260 people will die from lung cancer (86,930 in men and 72,330 among women), accounting for about 27% of all cancer deaths. Each year, more people die of lung cancer than of colon, breast, and prostate cancers combined.